The nitrogen
cycle represents one of the most important nutrient
cycles found in terrestrial ecosystems (Figure 9s-1).
Nitrogen is used by living organisms to produce a number of
complex organic molecules
like amino acids, proteins,
and nucleic
acids. The store of nitrogen found in the atmosphere,
where it exists as a gas (mainly N2), plays an important
role for life. This store is about one million times larger
than the total nitrogen contained in living organisms. Other
major stores of nitrogen include organic matter in soil and
the oceans. Despite its abundance in the atmosphere, nitrogen is often
the most limiting nutrient for plant growth. This problem occurs
because most plants can only take up nitrogen in two solid
forms: ammonium ion
(NH4+ ) and the ion nitrate (NO3- ).
Most plants obtain the nitrogen they need as inorganic nitrate from the soil
solution. Ammonium is used less by plants for uptake
because in large concentrations it is extremely toxic. Animals
receive the required nitrogen they need for metabolism,
growth, and reproduction by the consumption of living or dead
organic matter containing molecules composed partially of nitrogen.
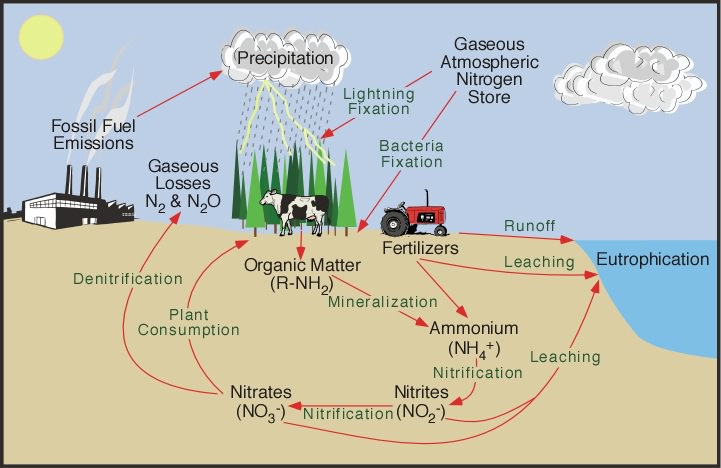
Figure 9s-1: Nitrogen
cycle.
In most ecosystems nitrogen is primarily stored in
living and dead organic
matter. This organic nitrogen is converted into inorganic
forms when it re-enters the biogeochemical
cycle via decomposition. Decomposers,
found in the upper soil layer, chemically modify the nitrogen found
in organic matter from ammonia (NH3 )
to ammonium salts
(NH4+ ). This process is known as mineralization and
it is carried out by a variety of bacteria, actinomycetes,
and fungi.
Nitrogen in the form of ammonium can
be absorbed onto the surfaces of clay particles in the soil. The
ion of ammonium has a positive molecular charge is normally held
by soil colloids.
This process is sometimes called micelle
fixation (see Figure 9s-1). Ammonium is released
from the colloids by way of cation
exchange. When released, most of the ammonium is often
chemically altered by a specific type of autotrophic bacteria (bacteria
that belong to the genus Nitrosomonas)
into nitrite (NO2- ).
Further modification by another type of bacteria (belonging to
the genus Nitrobacter)
converts the nitrite to nitrate (NO3- ).
Both of these processes involve chemical oxidation and
are known as nitrification.
However, nitrate is very soluble and it is easily lost from the
soil system by leaching.
Some of this leached nitrate flows through the hydrologic
system until it reaches the oceans where it can be returned
to the atmosphere by denitrification.
Denitrification is also common in anaerobic soils
and is carried out by heterotrophic bacteria.
The process of denitrification involves the metabolic reduction of nitrate (NO3- ) into
nitrogen (N2) or nitrous oxide (N2O) gas.
Both of these gases then diffuse into
the atmosphere.
Almost all of the nitrogen found in
any terrestrial ecosystem originally came from the atmosphere.
Significant amounts enter the soil in rainfall or through the effects
of lightning. The majority, however, is biochemically fixed within the soil by specialized
micro-organisms like bacteria, actinomycetes,
and cyanobacteria.
Members of the bean family (legumes) and some other kinds of plants
form mutualistic symbiotic relationships with nitrogen fixing bacteria.
In exchange for some nitrogen, the bacteria receive from the plants
carbohydrates and special structures (nodules) in roots where they
can exist in a moist environment. Scientists estimate that biological
fixation globally adds approximately 140 million metric tons of
nitrogen to ecosystems every year.
The activities of humans have severely altered the
nitrogen cycle. Some of the major processes involved in this alteration
include:
- The application of nitrogen fertilizers to crops has caused
increased rates of denitrification and leaching of nitrate
into groundwater. The additional
nitrogen entering the groundwater system eventually flows into
streams, rivers, lakes, and estuaries. In these systems, the
added nitrogen can lead to eutrophication.
- Increased deposition of nitrogen from atmospheric sources
because of fossil fuel combustion and forest burning. Both
of these processes release a variety of solid forms of nitrogen
through combustion.
- Livestock ranching. Livestock release a large amounts of
ammonia into the environment from their wastes. This nitrogen
enters the soil system and then the hydrologic system through
leaching, groundwater flow, and runoff.
- Sewage waste and septic tank leaching.